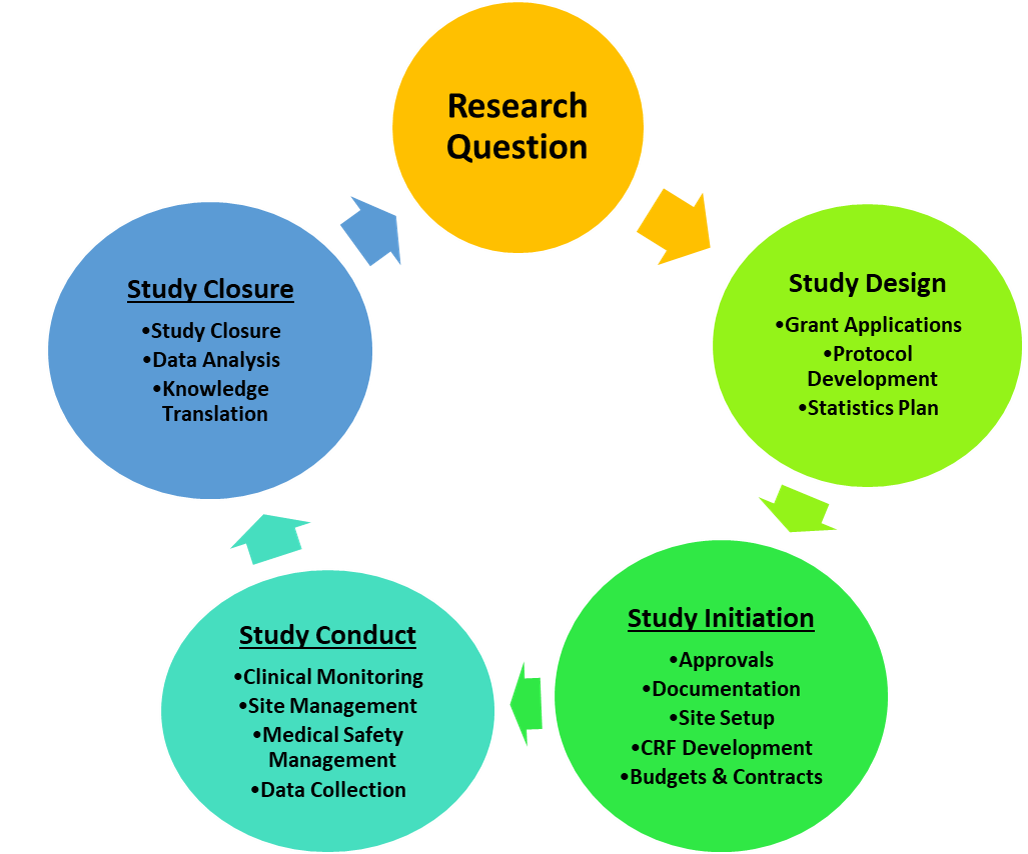
Study Design
We will ensure that your study optimally answers your research question, for example by honing the study design, methodology, and outcomes. We specialize in definitive randomized controlled trials – from tightly controlled to pragmatic – but also have expertise in pilot and feasibility studies, observational studies, and linkage to administrative health data. We provide customized services throughout the research process to meet the unique needs of each study.
1. GRANT APPLICATIONS
- Editing and internal peer review of application
- Planning project timelines, deliverables, and budget
- Ensuring contemporary topics (such as sex and gender; equity, diversity, and inclusion; knowledge translation; health economics) are considered for maximum impact
2. PROTOCOL DEVELOPMENT
- Write or edit study protocol under the guidance of an ACTU-affiliated Anesthesiologist
- Create study-specific documents (e.g., safety management plan, data monitoring plan, consent forms, manual of procedures)
- Adapt documents to country- or site-specific requirements (e.g., translation, regulatory requirements)
3. STATISTICAL PLAN
- Sample size calculation
- Developing a statistical analysis plan under the guidance of a PhD statistician
- Randomization code generations
Study Initiation
1. APPROVALS
- Prepare and perform submission to regulatory agencies (e.g., Health Canada, Clinical Trials Ontario) and ethics committees.
2. DOCUMENTATION
- Review or create all applicable study documents (e.g., standard operating procedures, case report forms, investigator brochure, delegation log, monitoring plan).
3. SITE SET-UP
- Perform site feasibility assessment, identify site leads and conduct Study Initiation Visits
- Develop site-specific study procedures (e.g., pharmacy procedures, recruitment procedures)
- Ensure study personnel are trained (e.g., through self-training, team trainings, posters, medical quality review meetings)
4. DATABASE DEVELOPMENT
- Develop data dictionary
- Prepare study completion guidelines
5. BUDGETS & CONTRACTS
- Budget development and financial management
- Develop, negotiate, and finalize contracts and agreements (e.g., confidentiality, service, financial)
Study Conduct
1. SITE MANAGEMENT
- Oversee data collection
- Resolve site-specific operational issues
- Communicate regularly with sites (e.g., via newsletters, meeting, etc. ) and provide follow-up on outstanding issues
- Resolve data queries
- Assist site staff to maintain study documentation
- Prepare & submit renewals and reports with regulatory agencies (e.g., Health Canada, Clinical Trials Ontario) and ethics committees
2. CLINICAL MONITORING
- Perform or oversee routine monitoring visits
- Perform routine unblinded pharmacy visits
3. MEDICAL SAFETY MANAGEMENT
- Perform patient data review
- Lead Independent Data Safety and Monitoring Committee meetings
- Review and report clinical database and protocol deviations
- Conduct comprehensive safety monitoring (SAE monitoring, reviewing, reporting)
- Provide investigators with new information relating to the continuing safe use of the Investigative Medicinal Product
- Submit and/or support expedited safety reports to regulatory agencies and ethics committees
Study Closure
1. Study Close-Out
- Perform site close-out visits
- Send notification to regulatory agencies and ethics committees
2. DATA ANALYSIS
- Conduct statistical analysis
- Write results sections, including figures and tables, for reports and publications
3. KNOWLEDGE TRANSLATION (KT)
- Assist with synthesis and dissemination of study results
- Tailor KT messages and strategies to specific audiences (e.g., care providers, patients, policy makers)